Green is the New Black: Super-hydrophobic Textiles & the Lotus Leaf
- Sophie O'Brien
- Jun 9, 2025
- 6 min read
Updated: Aug 18, 2025
If we are going by clichés, science and fashion don’t typically go hand in hand. But as a high school girl interested in science research and, truthfully, also curating my ideal wardrobe, I can attest to the contrary. While I’m not suggesting that lab coats should become the next trend, the fashion industry has, interestingly enough, become tangled up in materials science research to meet sustainability goals. The global fashion market is responsible for around ⅕ of the plastic produced worldwide and contributes 10% of carbon dioxide emissions. The fashion industry is one area of focus where scientists are attempting to find solutions to build a greener future, with those solutions lying in nature’s materials to prevent us from making the next fashion faux pas. Specifically, this blog post will be focused on the impacts of water-repellent fabrics in the clothing industry and their potential bioinspired solutions.

A Brief History
Fashion hasn’t always been unsustainable—I mean, just think of the way our ancestors made clothes out of natural fabrics for thousands of years, relying on both protein-based (animal) fibers like wool and cellulose-based (plant) fibers like cotton. However, in the 20th century, with the rise of industrialization, factory production of textiles became more favorable and efficient compared to the more expensive process of producing individually hand-made fibers. During this time came the reign of mass-manufactured synthetic fabrics (e.g., nylon, polyester, spandex), which are derived from the chemical cracking of petroleum to form polymers that are then heated and extruded into small fibers used in textiles. Among these, water-resistant fabrics treated with chemical coatings became especially popular. These hydrophobic (water-repellent) fabrics are useful for their utility in durability, weather resistance, industrial workwear, and more.
Synthetic Hydrophobic Fabrics: PFAS as “forever chemicals”
Many of you are most likely familiar with the term PFAS, which is short for polyfluoroalkyl substances. Or, if that’s still not ringing a bell, you probably know them by their catchier nickname: “forever chemicals.” Hydrophobic PFAS are renowned for their water-repellent and stain-resistant properties, and can be found in a wide range of applications, from clothing to non-stick pans to firefighting foams. The problem with PFAS is that they contain fluoropolymers such as Teflon (pictured below), which are highly resistant to microbial breakdown. As a result, hydrophobic fabrics from non-biodegradable PFAS pose huge environmental concerns as they persist indefinitely, contaminating waterways, ecosystems, and harming wildlife in the form of toxic microplastic waste.

The Lotus Effect: Defining Superhydrophobic
So, we are familiar with the term hydrophobic, but then how do we distinguish what superhydrophobic means? Let’s call on nature to help us explain!
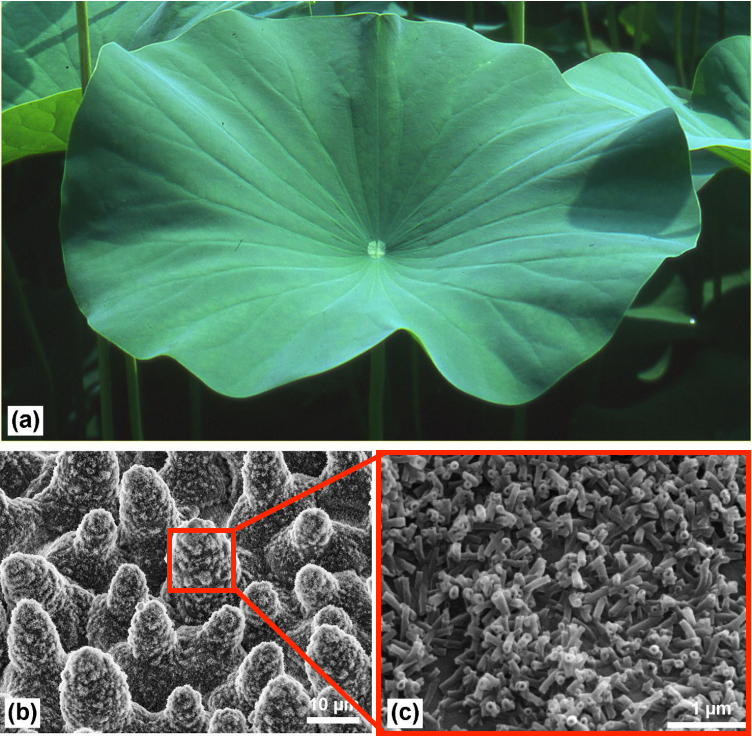
The lotus leaf is one of nature’s finest examples of a superhydrophobic surface. As you can see from the video, rainwater droplets either bounce off the lotus leaf or ball up in the center. The leaf stays completely dry. When the water runs off the leaf's surface, it carries any dirt along with it, meaning the leaf is in-fact self-cleaning!
Step one in learning about the superhydrophobic lotus leaf is understanding how surface energy works. Water forms a droplet shape when rolling off the lotus leaf to minimize its surface energy. Water molecules on the outside of a droplet—the ones in contact with external surroundings— are in higher energy states because they’re exposed at the liquid-air interface. On the other hand, the water molecules protected deep within the droplet—those not directly exposed to air—experience lower energy due to more favorable intermolecular forces. By taking on the shape of a sphere, the droplet is able to minimize its surface energy, since a sphere (fun fact!) has the lowest surface-area-to-volume ratio of any shape.
Although we may not usually take notice of it, water looks and behaves differently depending on the surface it makes contact with. Think back to when you spilled a glass of water on a wooden desk. Did it look the same as when you poured water on a freshly waxed car? No! That’s because different surfaces have different intermolecular interactions with water. This property is known as wettability. If water is attracted to the surface and spreads itself out, this is called wetting. If water beads up into droplets instead, then the surface is non-wetting.
The way liquids interact with surfaces can actually be summed up by a cool mathematical relationship: Young’s Equation! The contact angle, θ (theta), results from a balance of interfacial energies and geometric configuration.
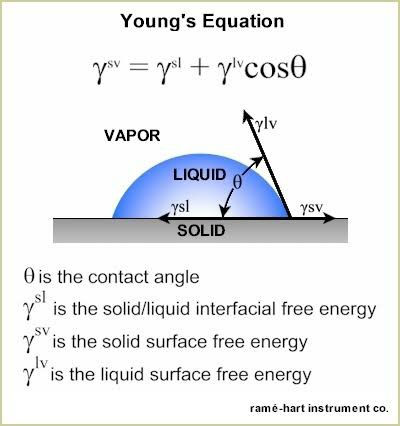
Low contact angle = favorable interactions → water spreads out.
High contact angle = repulsion → water beads up.
Now for the big reveal: a surface is considered superhydrophobic when the contact angle (θ) is greater than 150°. So now hopefully you see all that background information was necessary—if I had started with that definition, it wouldn’t have made any sense!
The lotus leaf achieves this >150° contact angle through its dual-scale surface structure. The surface of the leaf is littered with tiny microscopic bumps and nanostructures, which all are coated in a waxy, water-repellent substance. This rough micro/nano texture traps air and reduces the contact area with water, while the waxy coating simultaneously helps to provide low surface energy, causing water to easily roll off.
Bioinspired Solutions: Mimicking the Lotus Leaf
Now that we’ve explored how the lotus leaf gets its incredible superhydrophobic name, let’s see how scientists are working to reproduce these properties for textiles. The goal for scientists is to look beyond traditional chemical coatings to engineer fabric surfaces that, like the lotus, combine both microscopic surface texture and hydrophobic properties. If successful, this would allow for new sustainable and biodegradable textiles with high water contact angles and low sliding angles without utilizing harmful PFAS.
The first major strategy scientists are using is micro/nano surface texturing—a process that involves creating rough, uneven textures at the microscopic level that mimic the surface of a lotus leaf. For instance, researchers have developed techniques to grow billions of nanowhiskers—tiny fiber-like structures just 10 nanometers in size—directly on cotton fibers, which reproduces the effects of a highly uneven surface that minimizes the area water can touch. These nanowhiskers, can also be attempted to be etched on or use special spinning techniques to embed them onto a fabric's surface fibers. Excitingly, these surface modifications can be achieved using biodegradable materials on sustainable fabrics, introducing a future where we have 100% green options for clothing. One of these breakthrough pioneers is the company Cellulotech, who describe their method of nanosurface texturing as “attach[ing] bio-sourced molecules around cellulose fibers to make them insensitive to water.”
The second major approach is engineering non-toxic hydrophobic coatings. Instead of using the popular PFAS-based chemical treatments that come after the already harmful process of making synthetic fabrics, scientists are exploring alternative coating compositions with silicones, plant-based waxes, and biodegradable polymers. In some studies, nanoparticles of silicon are added to well-known hydrophobic polymers—such as polyethylene or polypropylene—to test their interactions and enhance their performance. When applied, these coatings form a nanoscale matrix on top of the fabric’s surface that can repel water and ultimately, keep the material dry. The company NeverWet’s coating, a design they themselves credit to the Lotus Leaf, managed to achieve contact angles of 160-175 degrees!
If you are curious to learn more, check out the company websites and/or sources that I used for this blog post below!
Chan, Chun Kit, Jooyoung Shin, and Shou Xiang Kinor Jiang. "Development of Tailor-Shaped Bacterial Cellulose Textile Cultivation Techniques for Zero-Waste Design." Clothing and Textiles Research Journal 36, no. 1 (2018): 33–44. https://doi.org/10.1177/0887302X17737177.
Getaneh, Seyoum A., Abdudin G. Temam, Assumpta C. Nwanya, Paul M. Ejikeme, and Fabian I. Ezema. "Advances in Bioinspired Superhydrophobic Surface Materials: A Review on Preparation, Characterization and Applications." Hybrid Advances 3 (2023): 100077. https://doi.org/10.1016/j.hybadv.2023.100077.
Liu H, Guo L, Hu S, Peng F, Zhang X, Yang H, Sui X, Dai Y, Zhou P, Qi H. Scalable Fabrication of Highly Breathable Cotton Textiles with Stable Fluorescent, Antibacterial, Hydrophobic, and UV-Blocking Performance. ACS Appl Mater Interfaces. 2022 Jul 18. doi: 10.1021/acsami.2c07670.
Rahman, Musfiqur. "Synthetic Fibers: Manufacturing Process and Classification." Textile Explainer, February 14, 2023. https://textileexplainer.com/synthetic-fibers-manufacturing-process-and-classification/.
Xing, Lili & Zhou, Qingqing & Chen, Guoqiang & Sun, Gang & Xing, Tieling. (2022). Recent developments in preparation, properties, and applications of superhydrophobic textiles. Textile Research Journal. 92. 3857-3874. 10.1177/00405175221097716.
Ye, Cuiying, Di Liu, Xiao Peng, Yang Jiang, Renwei Cheng, Chuan Ning, Feifan Sheng, Yihan Zhang, Kai Dong, and Zhong Lin Wang. "A Hydrophobic Self-Repairing Power Textile for Effective Water Droplet Energy Harvesting." ACS Nano 15, no. 11 (2021): 18172–18181. https://doi.org/10.1021/acsnano.1c06985.




Comments